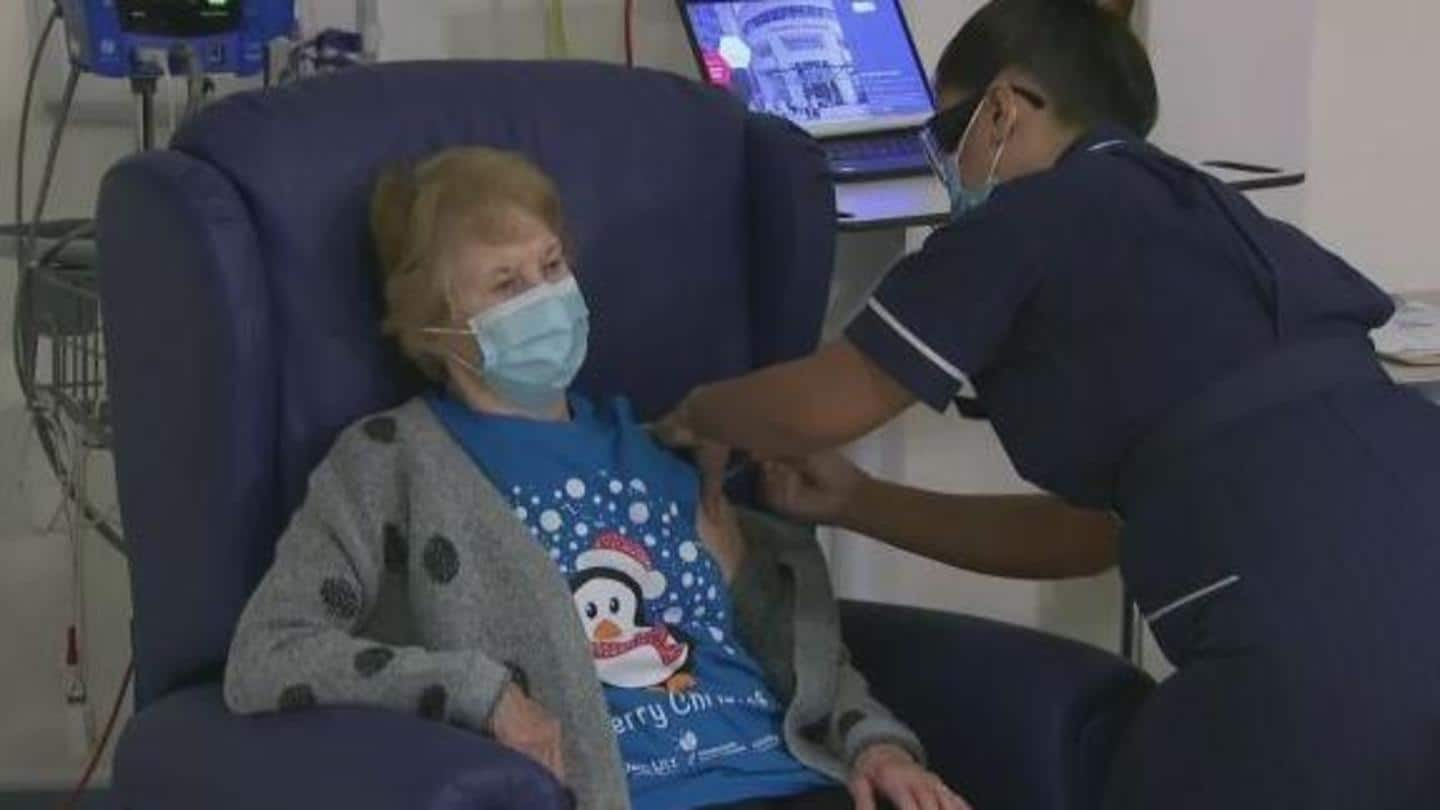
COVID-19 vaccine: 90-year-old woman receives first Pfizer shot in UK
What's the story
A 90-year-old woman in the United Kingdom has become the world's first person to receive the COVID-19 vaccine developed by Pfizer and BioNTech outside of clinical trials.
Last week, the UK had given emergency approval to the vaccine, called BNT162b2.
American pharmaceutical giant Pfizer and German drugmaker BioNTech have said the vaccine was found 95% effective in final trials.
Here are more details.
Details
I feel so privileged, says 90-year-old Margaret Keenan
Margaret Keenan reportedly received the vaccine shot at the University Hospital in Coventry, central England, at 6:31 am GMT.
Keenan—who turns 91 next week—said, "It's the best early birthday present I could wish for" as she can now spend time with her family and friends in the New Year.
"I feel so privileged to be the first person vaccinated against COVID-19," she said.
Vaccination
UK has ordered 40 million vaccine doses
The UK has become the first Western country to start vaccinating the general population.
It has placed orders for 40 million doses in total, enough to vaccinate 20 million people.
The government has secured some 0.8 million doses which will be administered in the coming weeks.
People aged above 80 and some health and care staff will be the first people to be vaccinated.
Health Secretary
Long march ahead of us, says Health Secretary
Meanwhile, UK Health Secretary Matt Hancock told BBC Breakfast that there was a "long march ahead of us but this marks the way out."
Feeling "conflicted," Hancock said that he was "thrilled and delighted" to see Keenan receive the first vaccine shot, but also felt "really determined that as a country we have got to stick together."
Vaccine
Pfizer's two-dose vaccine found 95% effective
Pfizer-BioNTech's vaccine BNT162b2 uses messenger RNA (mRNA) technology, which has never been used for an approved vaccine.
Late-stage trial data found the two-dose vaccine to be 95% effective. The efficacy was consistent across age, race, and ethnicity demographics, the companies said.
The UK had granted emergency approval to the vaccine on Wednesday, followed by Bahrain shortly after on Friday.
India
Pfizer has sought emergency approval in India
Further, Pfizer India has also submitted an application for emergency use authorization of the vaccine in the country.
However, India has indicated disinterest in procuring the vaccine since it requires low temperatures of -70°C, and maintaining such cold-chain facilities would be extremely difficult.
India has already pre-booked 1.6 billion vaccine doses, including 500 million doses of the vaccine developed by Oxford University and AstraZeneca.