Breakthrough method allows nanoscale imaging with conventional microscopes
What's the story
In a major breakthrough, researchers from the Massachusetts Institute of Technology (MIT) have developed a technique that expands tissue up to 20 times its original size for imaging. The method, which employs a standard light microscope, provides an affordable alternative to expensive super-resolution microscopes that are typically used for nanoscale imaging. The new approach could transform biology by making nanoscale imaging accessible to nearly any lab.
Microscopic view
A closer look at cellular structures
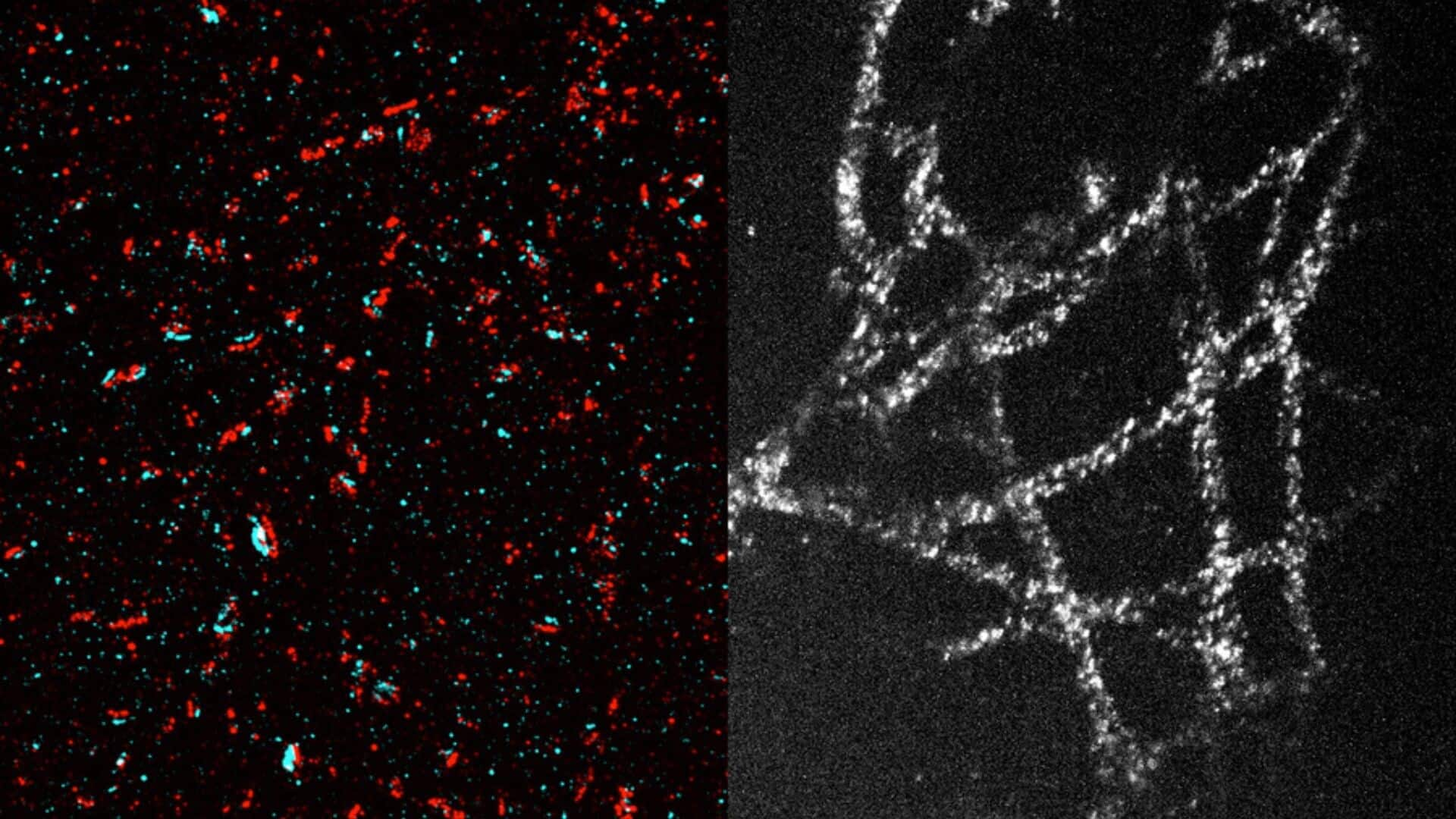
The innovative technique developed by MIT researchers allows for a resolution of some 20 nanometers. At this level of magnification, scientists can see organelles inside cells and protein clusters. The 20-fold tissue expansion allows a standard light microscope to achieve the resolution of high-end super-resolution microscopes, allowing scientists to examine cellular structures like microtubules and mitochondria in detail.
Gel innovation
The science behind single-step tissue expansion
In order to achieve a 20-fold tissue expansion in a single step, the MIT team had to create a gel that was highly absorbent and mechanically stable. For this, they used a gel made from N, N-dimethyl acrylamide (DMAA), and sodium acrylate. Unlike previous expansion gels that required an additional molecule to form crosslinks between polymer strands, this new gel forms crosslinks spontaneously and has strong mechanical properties.
Gel optimization
Optimizing the gel for enhanced expansion
The MIT researchers further improved the gel and its polymerization process to allow for a 20-fold expansion. They removed oxygen from the polymer solution before gelation, preventing side reactions that could disrupt crosslinking. This was achieved by running nitrogen gas through the polymer solution, replacing most of the oxygen in the system. After forming, select bonds in proteins holding tissue together are broken and water is added to expand it.
Practical use
Imaging applications of the new technique
Post-expansion, target proteins in tissue can be labeled and imaged. The MIT team successfully used this method to image tiny structures within brain cells, including synaptic nanocolumns - protein clusters arranged in a specific way at neuronal synapses. They also imaged microtubules and mitochondria in cancer cells, as well as individual nuclear pore complexes. Currently, they're using this technique to image glycans on cell surfaces that regulate cells' interactions with their environment.