Over 200 diabetics injured as app controlling insulin pump malfunctions
What's the story
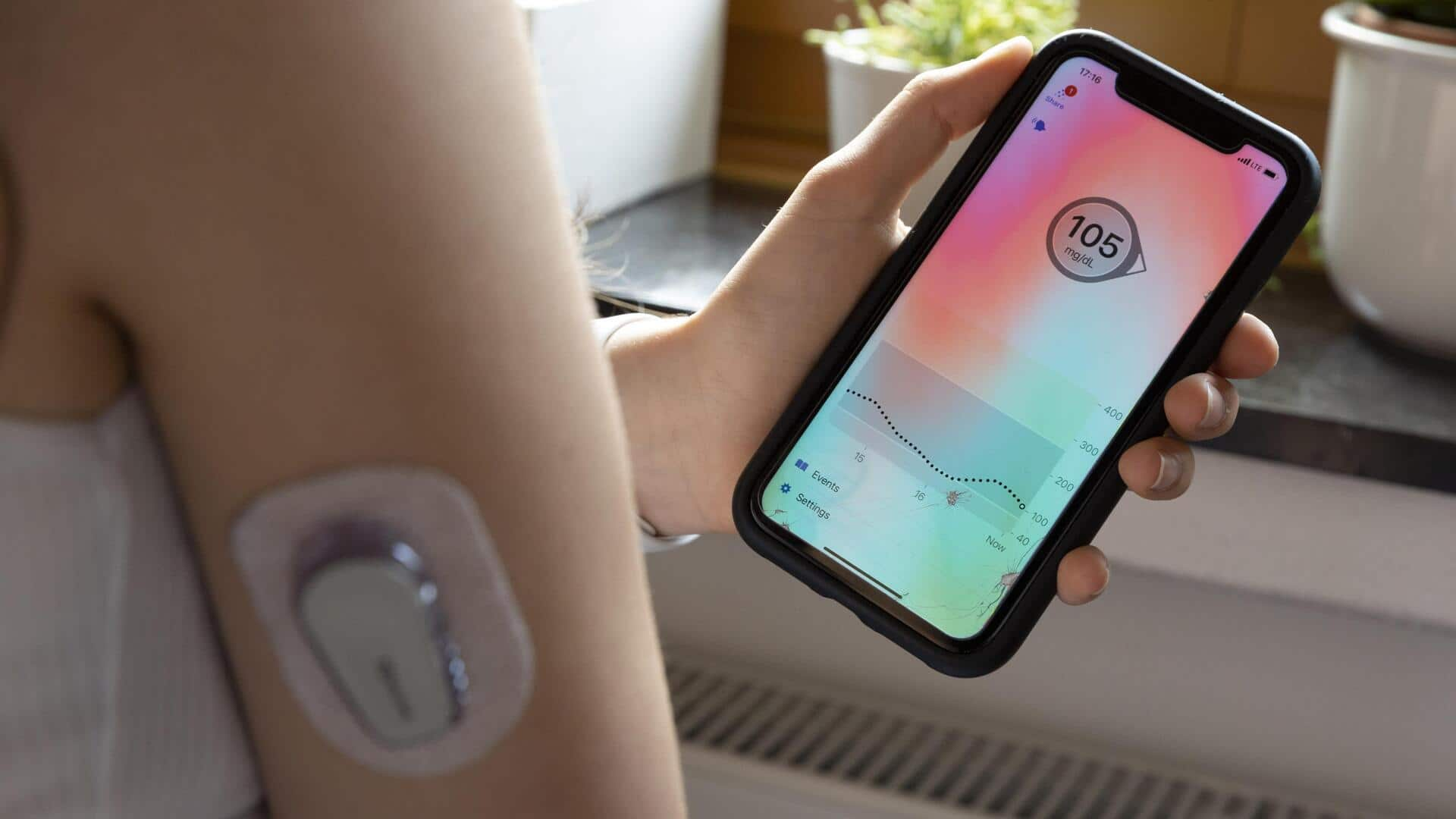
The US Food and Drug Administration (FDA) has issued a recall for version 2.7 of the t:connect app, an iOS application that controls insulin pumps for diabetes patients. The recall was prompted by a software glitch causing the app to crash and relaunch continuously on iPhones in a never-ending loop. This malfunction resulted in sending excessive Bluetooth signals to the paired t:slim X2 insulin pump, developed by Tandem Diabetes Care, thereby draining its battery rapidly, and causing over 200 injuries.
Recall classification
Recall classified as most severe due to potential risks
The FDA has classified this recall as a Class 1 — its most severe category — due to the potential risk of serious injury or death from using the faulty pumps. The recall pertains solely to the software update and does not necessitate the return of devices. The malfunction has affected nearly 86,000 devices, highlighting the severity of its impact on insulin delivery.
Health risks
Malfunction leads to life-threatening condition in diabetes patients
The FDA press release stated that a pump shutdown would suspend insulin delivery, potentially leading to an under-delivery of insulin resulting in hyperglycemia or diabetic ketoacidosis. This condition can be life-threatening because of high blood sugar and lack of insulin. In the US, over 38 million people have some form of diabetes and about 350,000 people use an insulin pump for treatment. These vital pumps consistently inject essential hormones into the body, replacing the need for manual injections.
Pump failure
Tandem's insulin pump failure results in 224 injuries
Tandem's t:slim X2 pump, which is controlled by the faulty app, has failed significantly with 224 injuries reported by the FDA. Tandem reported 81 "adverse events," including one injury needing medical intervention. Despite pumps releasing a low power alert and an alarm prior to shutdown, it may already be too late if a user does not have a charger readily available. A shutdown during sleep could be fatal.