Are you eligible for COVID-19 vaccination? Should you get it?
What's the story
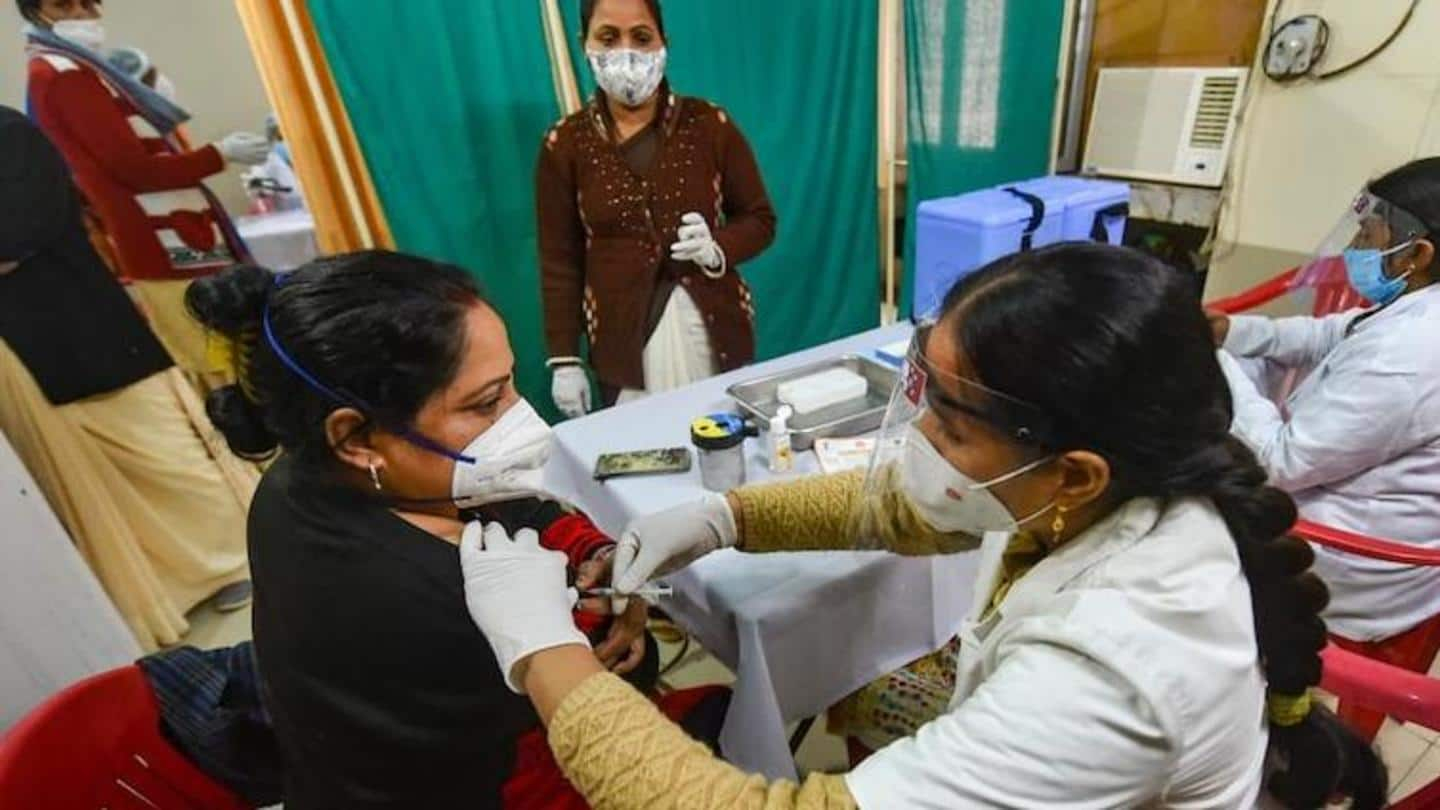
India is scheduled to begin the second phase of its COVID-19 vaccination drive from March 1. The first phase—that aimed to vaccinate 3 crore health and frontline workers—was launched on January 16. In the second phase, the government is aiming to inoculate 27 crore people who are above the age of 60 and people with co-morbidities who are above the age of 45.
Eligibility
Who is not eligible for the vaccine?
People under the age of 18 are not allowed to receive the COVID-19 vaccine. Currently, India is using two vaccines: Serum Institute of India's Covishield and Bharat Biotech's COVAXIN. Those with allergic reactions to vaccines, pharmaceutical products, notable food allergies also cannot get the vaccine along with pregnant/lactating mothers and those who have had adverse reactions to a COVID-19 vaccine earlier.
Eligibility
Who is temporarily not eligible for the vaccine?
People showing active symptoms of a COVID-19 infection. COVID-19 patients who have received anti-SARS-CoV-2 monoclonal antibodies or convalescent plasma. Acutely unwell and hospitalized patients (with or without intensive care) due to any illness. Separately, for people with a history of any bleeding or coagulation disorder (platelet disorder, clotting factor deficiency, or coagulopathy), the vaccine should be administered with caution.
Adverse events
What are the common adverse events with Covishield?
Covishield may trigger mild adverse events such as injection site tenderness; injection site pain; headache; fatigue; myalgia (muscle pain); discomfort; pyrexia (an abnormal elevation of body temperature); chills; and nausea. Paracetamol is advised in such cases. "Very rare events of demyelinating disorders" have also been reported after vaccination with Covishield, albeit "without the causal relationship establishment."
Information
What are the common adverse events with COVAXIN?
Vaccination with COVAXIN may lead to adverse events such as injection site pain, fatigue, fever, headache, body ache, nausea, abdominal pain, dizziness, sweating, cold, and cough. According to Bharat Biotech, no serious adverse events were reported in Phase 1 and 2 trials.
Instructions
What are the general instructions on administering vaccine?
The double-dose vaccines Covishield and COVAXIN are not interchangeable. The second dose of the vaccine will have to be the same as the first dose. Vaccinators must store both vaccines at +2°C to +8°C and protect them from exposure to light. If the vaccine in the vial is found to be frozen, it should be discarded before administering.
Information
What other things should you keep in mind?
The efficacy of COVID-19 vaccines "may be less" in individuals with co-morbidities. Further information on the subject is awaited. If you experience any side-effects after being administered the vaccine, it is advisable to contact your physician immediately.